Breaking News
Science and Technology
Revolutionary Digital Health Solution: The Cordella PA Sensor Triumph
Lauren Miller
March 5, 2024 - 23:05 pm

Breakthrough in Heart Failure Management: Endotronix's PROACTIVE-HF Trial Paves Way for FDA Approval
NAVERVILLE, IL, March 5, 2024 – Endotronix, Inc., a leading company operating at the nexus of digital health and medical technology, proudly revealed the six-month results from its pivotal PROACTIVE-HF trial. The trial is a critical step toward transforming the care of heart failure (HF) patients. Endotronix’s Cordella Pulmonary Artery (PA) Sensor, the focal point of the trial, showed substantial promise for patients with New York Heart Association (NYHA) class III HF, leading to significant reductions in hospitalizations due to heart failure. This marks an encouraging stride in the quest for pre-market approval (PMA) from the U.S. Food and Drug Administration (FDA).
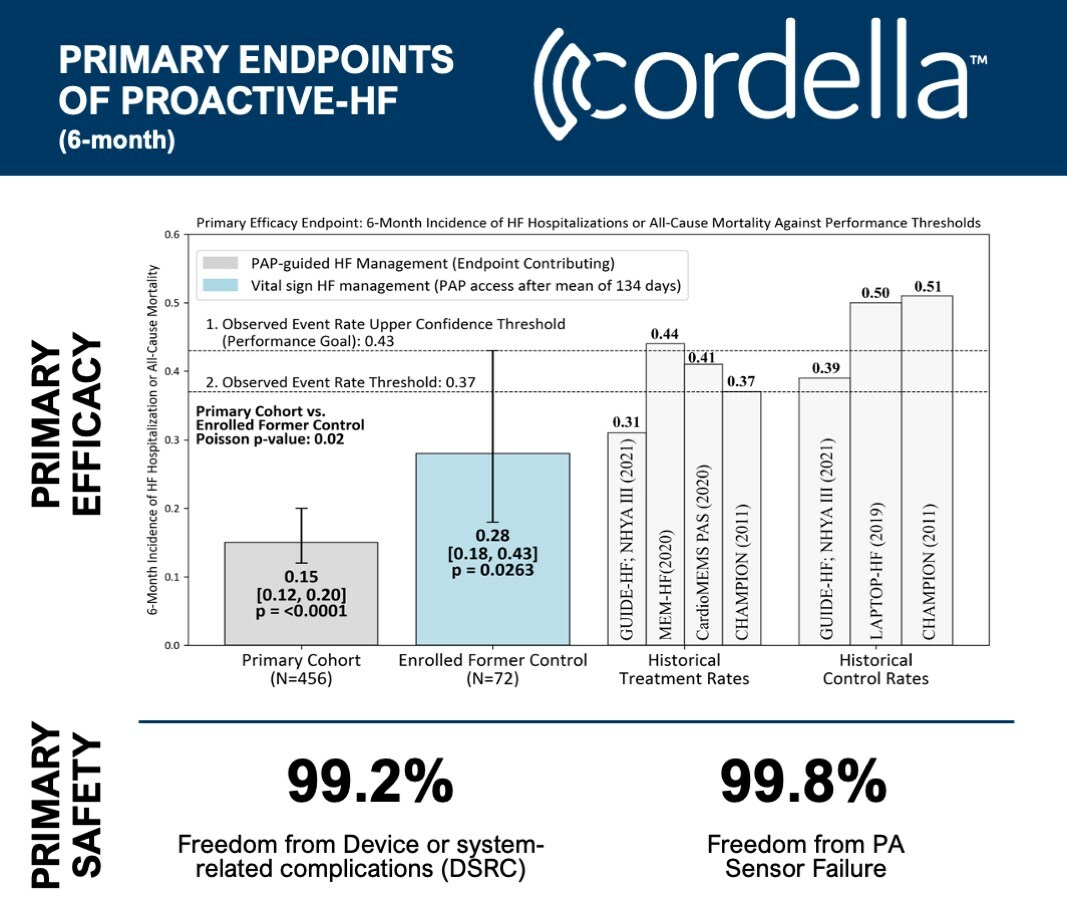
The trial's primary safety and efficacy outcomes indicate successful achievement with remarkably low rates of heart failure hospitalizations. Moreover, patients showed considerable enhancement in their quality of life, a spike in physical activity, and improvement of NYHA functional class status.
Dr. Liviu Klein, a distinguished cardiologist and key proponent of the study, presented these impactful findings at the Cardiovascular Research Foundation's Technology and Heart Failure Therapeutics (THT) conference in Boston, MA.
Exceptional Data Indicate a Bright Future for Heart Failure Patients
The exhaustive PROACTIVE-HF trial was a multi-center and prospective study spanning across 75 sites in the United States and Europe, where 528 individuals with NYHA class III HF were implanted with the Cordella PA Sensor. The cohort included over half the implanters specialized in heart failure treatment.
The compelling six-month single-arm results demonstrated not only the primary safety and efficacy endpoints but also several clinically significant secondary efficacy endpoints:
- 99.2% Freedom from Device or System-related Complications (DSRC)
- 99.8% Freedom from PA Sensor Failure
- Only a 0.15 rate of HF hospitalization/all-cause mortality at six months, starkly lower than the target of 0.43 based on preceding PA pressure-guided HF management trials (p<0.0001)
- Primary efficacy met across all PA pressure-guided HF management sub-groups (p<0.0001)
The granularity of the improvements were presented in detail:
- A 5-point uplift in the Kansas City Cardiomyopathy Questionnaire (KCCQ) scores (p<0.0001)
- A 23 meter advance in the six-minute walk test (6MWT) (p=0.001)
- 144 patients climbed up one NYHA class designation (p<0.0001)
- A 2.4 mmHg drop in seated mean PA pressure from the baseline for congested patients (p=0.001)
- A significant reduction of 5.9 mmHg in in-office systolic blood pressure from baseline (p<0.0001)
- A weight reduction by 2.2 pounds in at-home measurements (p=0.001)
These awe-inspiring findings highlight the efficacy of the Cordella PA Sensor and are in alignment with the earlier 12-month outcomes of the SIRONA 2 trial.
To access the complete PROACTIVE-HF trial results, navigate here.
Pioneering Patient-Centric Cardiac Care
Dr. Liviu Klein praised the trial results as a testament to heart failure management evolution. He emphasized the Cordella system's patient-centric approach, combining a user-friendly handheld PA pressure reader and patient access to their own health data, fostering a partnership in their care journey. Patients, armed with actionable data, were propelled to make healthier lifestyle choices. The clinicians, equipped with a holistic view of patient health status, adeptly modulated medications, which culminated in outstanding patient outcomes. The study's high patient compliance (greater than 6 average weekly Cordella submissions) and clinician engagement (over 2 average weekly reviews) were significant contributors to these outcomes.
Comprehensive Remote Care Technology Awaits FDA Green Light
Harry Rowland, CEO and co-founder of Endotronix, took pride in the company's and its partners’ relentless pursuit of superior care for heart failure patients. Enthusiastically, he envisions the infusion of proactive and comprehensive care through Cordella. This innovative approach enhances patient lives, supported by strong evidence from PROACTIVE-HF. Looking ahead, the anticipated commercial launch later in the year signifies a pivotal moment for clinicians who aim to empower patients with heart failure to lead robust, active lives.
Furthermore, Endotronix is cementing Cordella's clinical credentials with the ongoing PROACTIVE-HF 2 trial, expected to underscore the technology's potential further.
Endotronix: At the Vanguard of Medtech and Digital Health Innovation
Endotronix stands at the forefront of revolutionizing heart failure care through its pioneering medtech and digital health solutions. The Cordella solution orchestrates proactive, data-driven heart failure management, fostering patient engagement and decongestion to ameliorate outcomes. At its core, the Cordella Sensor, an implantable pulmonary artery (PA) sensor, meticulously measures congestion indicators to permit early targeted therapy interventions.
The Cordella Heart Failure System is a holistic platform for patient health management, aggregating comprehensive vital sign data from non-invasive devices into a single, coherent system that bolsters patient and clinician collaboration. With trended insights, Cordella is designed to seamlessly integrate into current clinical workflows, elevating guideline-based care across the entire spectrum of heart failure care.
Discover the full potential of Cordella at www.endotronix.com.
Please note, the Cordella Pulmonary Artery Sensor System is considered an investigational device and currently lacks approval for clinical use across all geographies. It remains restricted by Federal (United States) law to investigational use and is solely for clinical investigation. Conversely, the Cordella Heart Failure System, sans the sensor, has obtained commercial clearance in both the U.S. and E.U.
Toward a Future with Trustworthy Advanced Therapeutics
The press release constitutes forward-looking statements, which include predictions, estimates, and other forward-focused information. These statements should not be regarded as an iron-clad commitment to future performance since they are not definitive guarantees.
View the detailed graphic data related to the PROACTIVE-HF clinical trial at PROACTIVE-HF Data PR Image.
For a glimpse of the innovative spirit of Endotronix, view their corporate logo at Endotronix Logo.
In Summary: A New Dawn in Heart Failure Intervention
As the medical community waits with bated breath for the FDA's review of the PMA application, the results of the PROACTIVE-HF trial have painted a promising picture for the future of heart failure management. The initiative demonstrated by Endotronix in pioneering technologies that meld medtech prowess with digital health insights heralds a new era in cardiac care, emphasizing patient empowerment and remote management efficacy.
Endotronix's success in meeting primary safety and efficacy endpoints signifies a potential paradigm shift not just in heart failure patient outcomes but in the way we approach and manage chronic diseases. With the healthcare landscape increasingly pivoting toward proactive care delivery, digital health solutions like the Cordella System are poised to play a critical role in shaping a new standard in patient care.
High Compliance and Clinician Engagement: A Formula for Success
The proactive patient engagement strategies and the clinical benefits demonstrated in the PROACTIVE-HF trial underscore the importance of innovative remote care technologies. The trial’s high patient compliance rate is indicative of the system's ease of use and acceptability among patients, a crucial factor in the long-term management of chronic conditions like heart failure.
Clinician engagement also played a pivotal role, with frequent reviews of patient data ensuring timely interventions. Such a multifaceted and interactive approach exemplifies best practices in patient-centered care, promising a future where technology and high-touch medicine work hand in hand to improve patient outcomes.
Impediments Overcome and Clinical Proof Fortified
In advancing toward the larger goal of full market access and affecting real change in patients' lives, Endotronix has navigated the regulatory landscape effectively. By compiling a robust clinical data package and proactively seeking FDA’s nod through the PMA application, Endotronix has laid a grounded foundation for their Cordella PA Sensor System not just as a novel device, but as a clinically validated solution for heart failure management.
Anticipating a Transformative Horizon for Heart Health
In essence, the PROACTIVE-HF trial epitomizes a transformative step in heart failure care. The proactive use of the innovative Cordella PA Sensor System has proven its worth in clinical trials, setting the stage for potentially reshaping heart failure management. Provided the FDA's review results in approval, clinicians and patients alike may soon witness the arrival of a technology that can redefine expectations for heart health and quality of life.
As Endotronix forges ahead, the potential for improving the lives of those living with heart failure continues to expand with the promise of innovative therapies becoming available. The vision of seamless, integrated care facilitated by Cordella's advanced technology platforms is within grasp, signifying a new chapter in the standard of care for heart failure patients across the globe.
In closing, this is more than just trial data; this is about the potential future where heart failure management is fundamentally enhanced by technology. Endotronix's journey showcases a steadfast commitment to clinical excellence and a brighter outlook for heart failure patients worldwide—an outlook defined by innovation, empowerment, and a relentless pursuit of health and wellness.
health guardian plus© 2025 All Rights Reserved




















